Bringing New Drugs to Market More Quickly and Efficiently
Typically, it takes ten years for a new medicine to make it to the marketplace in the U.S., with the average cost of researching and developing a drug estimated at $2.6 billion. The likelihood of a drug being approved after clinical testing is still less than 12 percent, according to the Congressional Budget Office. But with the onset of the COVID-19 pandemic, many people – including those in pharma and biotech, regulatory agencies, patients, and the general public – began to rethink key assumptions about drug development.
Despite enormous challenges, the United States Food and Drug Administration (FDA) found ways to partner with pharma and biotech innovators to accelerate the approval of new drugs to treat COVID-19. Under emergency use authorization, the FDA approved the use of SARS-COV-2-targeting monoclonal antibodies, antiviral drugs, immune modulators, sedatives, and renal replacement therapies. It’s unfortunate that it took a global pandemic to show it’s possible to safely and responsibly speed up the approval process, but if drug development can continue to become more streamlined, new therapies could be introduced at faster rates – potentially improving the lives of millions.
New Technology Can Help Bring Novel Therapies to Market Sooner
New technology can play a significant role in reducing the costs and increasing the speed of bringing novel therapies to market, without compromising safety standards. Right now, efficiency-enabling opportunities such as increased automation during manufacturing help reduce time and costs in bringing new drugs to market. It can take six months or longer for drugs to even get in line for production, so many pharma companies are looking for ways to speed up the process without sacrificing quality.
Cambrex, a pharma contract development and manufacturing organization (CDMO), is one example of a company that’s reducing lead time to get drug candidates into trials and commercialized. The company recently completed a $50 million expansion of its active pharmaceutical ingredient (API) manufacturing space in Iowa, which supports both batch and flow chemistry processes. The facility allows for drugs that are made at the clinical trial level in small scale to then be made at the same facilities in higher volumes for commercial supply – reducing inefficiencies in finding different facilities and vendors for larger scale production.
Quotient Sciences, another Permira fund portfolio company, provides fully integrated pharmaceutical drug development and manufacturing services that are focused on reducing the time and cost of bringing new drugs to market. Their unique flagship platform - Translational Pharmaceutics®, integrates drug substance, drug product and clinical testing activities all under a single organization. This streamlined approach increases the likelihood of clinical and commercial success and has been proven to reduce both R&D costs and development times by more than 12 months. Quotient’s continued investment in their global facilities and technologies, including the latest in processing equipment, ensure their client’s drug programs move along as quickly as possible from the candidate development stage through to commercialization.
Clinical Trials: The Traditional Model Evolves
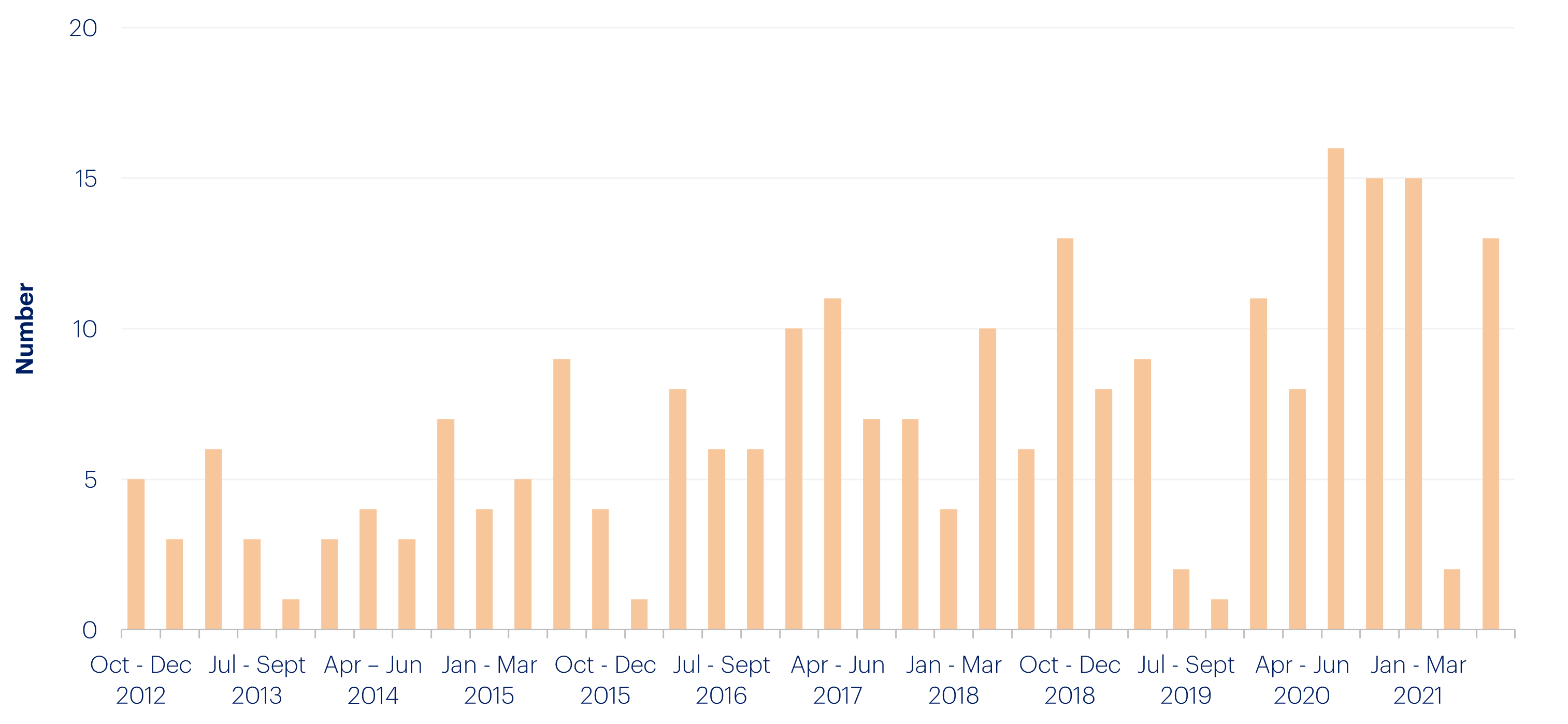
The clinical trial process in the U.S. is notorious for being thorough, but slow, with trials usually taking six to seven years. Getting all the necessary approvals from internal review boards, clinical trial participants, and the FDA is a laborious and time-consuming process. But it doesn’t have to be. The FDA originally introduced the Fast Track process in order to expedite approval for drugs that treat serious conditions and fulfill unmet needs, reducing the length of the clinical trial process. In 2020-21, the FDA’s emergency use authorization of COVID-19 treatments proved the approval process for new drugs could continue to evolve to meet new challenges.
Number of Fast track designations granted in the quarter:
Moving Forward with Drug Development
Pharma and biotech companies are competing to bring effective new therapies to market quickly to treat, diabetes, cancer, heart disease, and more. A change in beliefs about what’s possible, combined with the opportunities new technologies afford, means now is the time to quickly develop even more novel therapies so they’ll be ready when they’re needed most.